25+ Calculating Excess Reactant
Multiply the original amount of magnesium hydroxide 65 by 704 percent to find the amount of magnesium hydroxide used. HCl NaOH NaCl H 2 O.

Ch 9 Notes Stoichiometry Ppt Video Online Download
To find out how much excess remains I use a stoichiometric setup where I find how much of the excess reactant is used based on if all of the limiting reactant is used.

. To find the amount of remaining excess reactant subtract the mass of excess reagent. Two aqueous solutions one of 05 M HCl and 08 M NaOH can be. Use the molar ratio from the equation to convert moles of O2 from Step 3 to moles of NH3 and then convert moles of NH3.
A crucial skill in evaluating the conditions of a chemical process is to determine which reactant is the limiting reactant and which isare the excess reactants. Work out 65 x 0704 4578. The coefficients of a balanced.
That can be done by calculating the amount of product that can be formed from given amounts of two reactants and choosing the smaller number. 12M views 6 years ago. For the above reaction Molecular mass of Na 23g Molecular mass of Cl 2 2 x 355 71g This 2371 is a.
That is the theoretical yield. It helps to get an idea on the quantity of product formation and percentage of yield calculation. It shows you how to perform stoichiometric calculations and.
Convert mass of each starting reactants to moles. Using the amount of one reactant at a time find the amount of product formed. CALCULATING THE AMOUNT OF AN EXCESS REACTANT Cisplatin an anticancer agent used for the treatment of solid tumors is prepared by the reaction of ammonia with potassium.
How to find limiting reagent and excess reactant for the limiting reactant equation given below. Calculate the mass of excess reactant used up. N2 H2 NH3 Solution.
Identifying limiting reactant and excess reactant is very important for a chemical reaction. As the given reaction is not balanced so its balanced form is as. This online Chemical Reaction Calculator checks whether a given chemical equation is balanced and finds the appropriate stoichiometric coefficients.
Begin with a balanced chemical equation and starting amounts for each reactant. To calculate the excess reactant firstly we will balance the chemical reaction. 2Na sCl 2 g2NaCl s Then we will calculate the molecular mass of each reactant.
How to Find Excess Reactant Assume a reaction that commences with 10 moles of hydrogen gas H2 H 2 and 7 moles of oxygen gas O2 O 2. This chemistry video tutorial shows you how to identify the limiting reagent and excess reactant. Find the limiting reagent and quantity of excess.
The reactant that gave the smallest amount of product is the limiting reagent and the amount produced is the. The reactant that produces a larger amount of product is the excess reagent.

Stoichiometry Limiting Reactant Left Over Excess Reactant Percent Yield Study Chemistry With Us Youtube

Finding The Amount Of Excess Reactant Left Over Ppt Download

Calculating The How Much Excess Reactant Remains Youtube
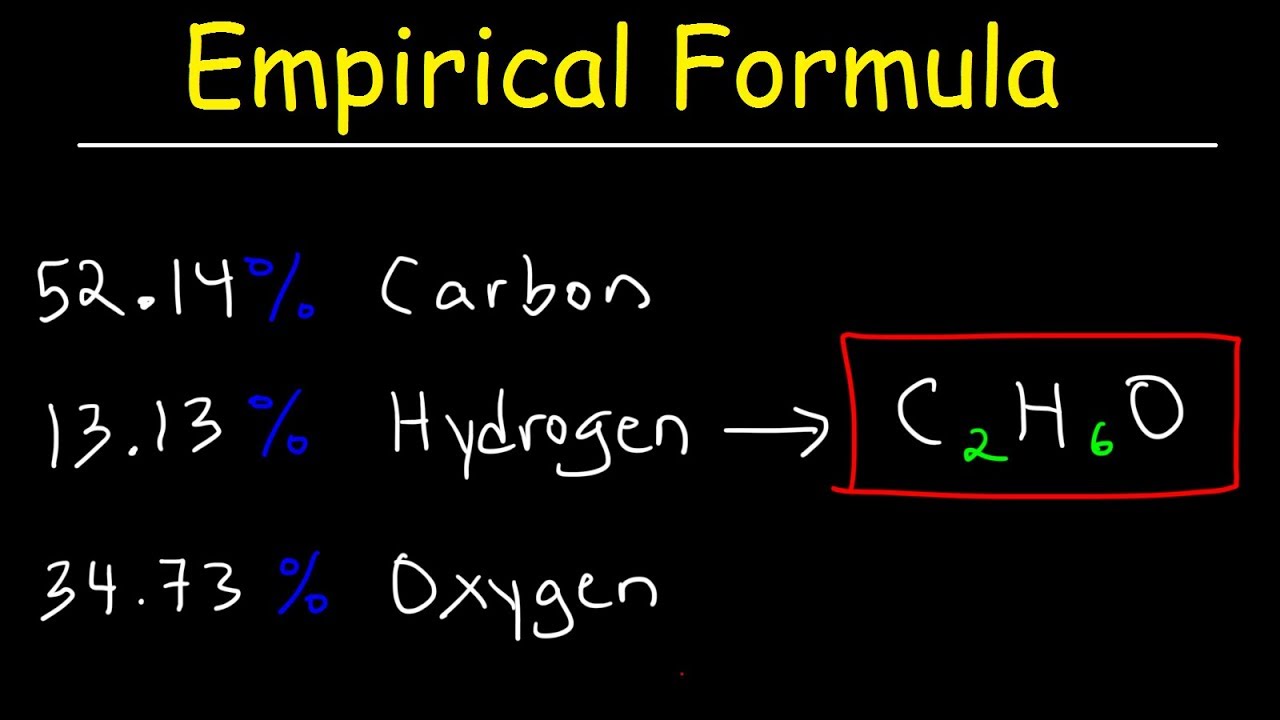
Stoichiometry Limiting Excess Reactant Theoretical Percent Yield Chemistry Youtube
What Is The Number Of Millimoles Of Hcl Required To Neutralize 10 Ml Of 0 2 M Na2co3 Quora
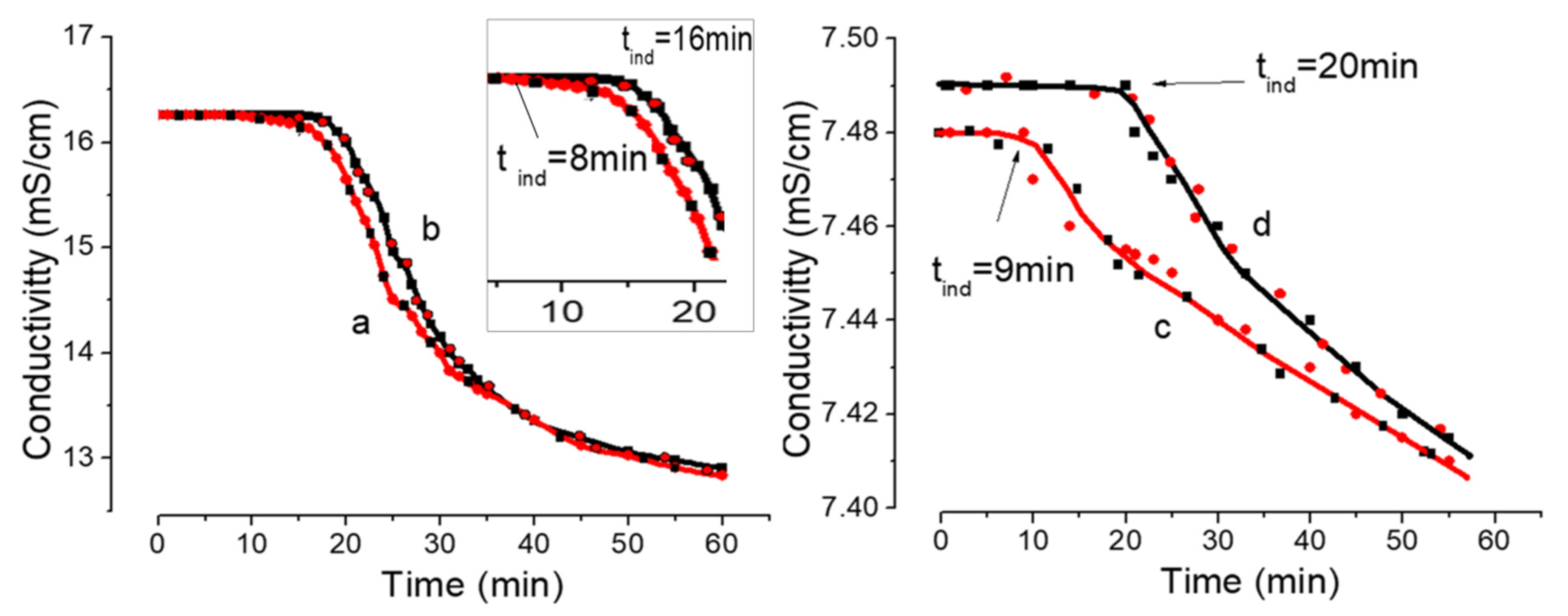
Minerals Free Full Text Initial Stages Of Gypsum Nucleation The Role Of Nano Microdust

4 2 Limiting Excess Reagents Chemistry Libretexts

Percent Excess Air Combustion Youtube

Modeling Of Transport Phenomena In Fixed Bed Reactors For The Fischer Tropsch Reaction A Brief Literature Review

Pdf Chapter Two Saif Ali Academia Edu

Calculating Mass Of Excess Reactant Leftovers In Limiting Reactant Problems Tutorial Youtube
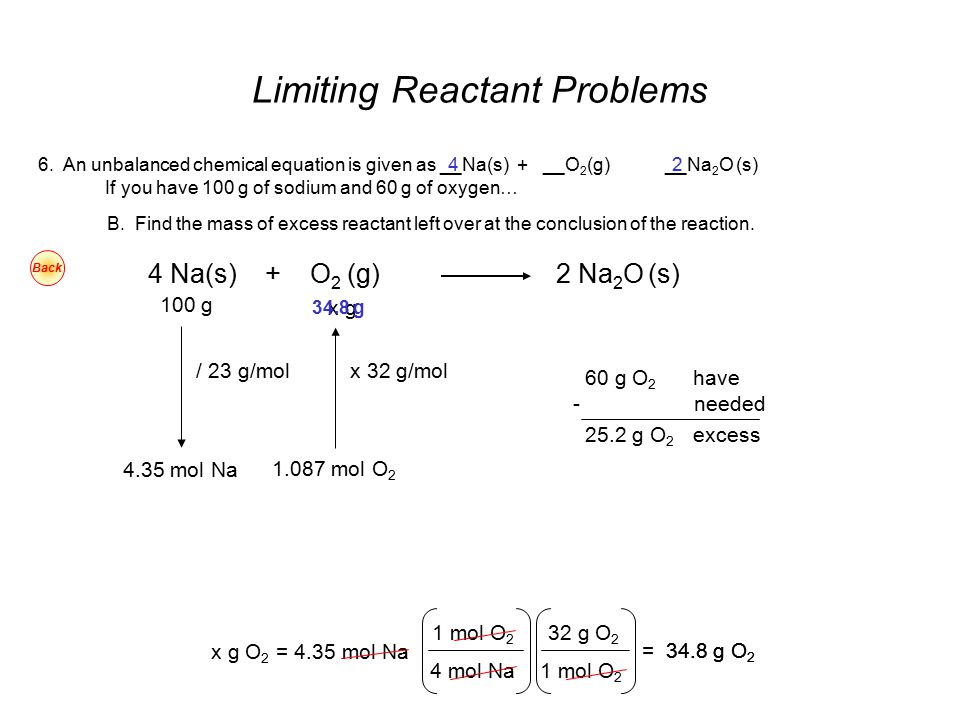
Excess Reactant Ppt Video Online Download

Chapter 3 Mass Balance Balance On Reactive Processes System Ppt Video Online Download
What Are The Calculations Needed To Find The Amount Of Excess Reactant Quora

Stoichiometry Limiting Reactant Left Over Excess Reactant Percent Yield Study Chemistry With Us Youtube

Limiting And Excess Reactants In Chemistry

Limiting And Excess Reactant Stoichiometry Problems Youtube